
Review of the Buddleja x weyeriana hybrids and future prospects for the introgression of yellow flower colour into Buddleja davidii
Updated February 2023.
Introduction
Horticulturalists have always relished the prospect of creating new plants by the hybridisation of geographically separated species. One of the earliest Buddleja hybrids was what became known as B. x weyeriana. During WW1 van de Weyer of Smedmore House, at Corfe Castle, pollinated the South American species B.globosa with pollen from B.davidii var. magnifica, a native of China (van de Weyer 1920). His F1 plants were mostly greyish-white, intermediate between the two species and somewhat of a disappointment, but the F2 generation (assumed to be a cross of two F1 plants) was much more successful. Two of these, B. x weyeriana 'Golden Glow' and 'Moonlight', are still popular today and most other B. x weyeriana cultivars are most likely sports from these original plants, as is the case with B. x weyeriana 'Sungold' (Fig.1) (De Vogel 1967).
Although the current B.x weyeriana yellow hybrids are attractive and popular garden shrubs all have ball-shaped heads on an interrupted panicle. The race is on to produce a new hybrid that more closely resembles B. davidii with long uninterupted panicle inflorescences and a clear yellow colour. Such a plant would have a high commercial value in the horticultural trade.

Fig. 1: B.x weyeriana 'Sungold' shows the typical inflorescence of these hybrids.
Buddleja globosa and Buddleja davidii

Fig 2a: A 1906 illustration of B. variabilis var. Magnifica (B. davidii), the cultivar or variety used by Weyer in his hybridisation.
What about the (grand-)parent species? Both are hardy in the UK and very popular in gardens. B. davidii is hermaphrodite, tetraploid (chromosome number of 76) and from China. The plant used by van de Weyer is an early cultivar (or natural variety) he describes as B. variabilis var. Magnifica, a violet-coloured B. davidii. Normally it would flower from July in the UK and the inflorescences form long conical panicles with several hundred individual perfect flowers (Fig.2a). Modern cultivars often have larger flower spikes but this variety would have been the best available to van de Weyer, as his hybridisations pre-date virtually all the known cultivars.

Fig 2b: A modern B. davidii cultivar with a very long panicle.
B.globosa is dioecious, diploid (chromosome number of 38), and a native of the Chilean Andes. It is a common belief that it is hermaphrodite like B. davdii but in common with most New World Buddlejas individuals are either male or female. The exception is the Stachyoides series, e.g. B.tubiflora, which are frequently hermaphrodite; there are a also few trioecious species (a population which can be male, female and hermaphrodite). The confusion arises with B. globosa because the flowers appear to have both anthers and a pistil, although only one or the other is functional: either the anthers or ovary are empty. This is called cryptic dioecy or occasionally micro-dioecy. It is a difficult to correctly identify the genders and often requires dissection, staining and microscopic examination of the flowers. For the amateur horticulturalist, simple observation may suffice to identify the gender of a B. globosa plant providing there is a suitable pollinator in the vicinity: the females will produce seed pods and the males never will. Dioecy has evolved as a means to facilitate out-crossing and is a relatively simple mechanism compared to the evolution of self-incompatibility in monoecious and hermaphrodite species (Norman 2000).

Fig. 3: B. globosa inflorescence and, inset, a mature seed pod.
B. globosa normally flowers in May/June and only rarely overlaps the flowering period of B. davidii so van de Weyer was fortunate indeed to manage this cross. Fortunate too, in that he happened to own a female plant. The inflorescences consist one terminal and several pairs of pedunculate globose heads, each about 1.0 - 3.0 cm in diameter and with 30 - 50 flowers. Although the gross structure of the inflorescence is different to B.davidii, the individual flowers are quite similar except the corolla tube is reduced in B. globosa.
Flower Colour in Buddleja
The purple/lilac flower pigments in the corolla of Asian Buddlejas are presumably anthocyanins as in other members of Scropulariaceae. Anthocyanins are just one type of flavonoid; other flavonoids include flavones and aurones. All are derived from the same precursors called chalcones (fig 4), which are themselves yellow if they accumulate in the flower's cells. Flavones can be yellow or colourless (but reflecting near-UV wavelengths so visible to insects) and are often co-pigments. Floral flavones rarely give a bright yellow colour but are more likely to appear cream or pale yellow. Aurones are a less common class of flavonoid restricted to a few plant families including Antirrhinum (formerly Scrophulariaceae, now assigned to Plantaginaceae) and are responsible for a bright yellow colour (Asen et al, 1972). In opened flowers the level of the flavones can be five to ten times higher than that of the anthocyanins or aurones (Ono et al 2006).
However, it is common for yellows and oranges to be the result of chemically different pigments: carotenoids. The yellow pigment in the centre (throat) of Asian Buddleja flowers is non-cyclic crocetin-digentobiose ester (a carotenoid) (Tallent-Halsell and Watt, 2009), much the same as the pigment as in saffron. Flavones are also known to be present in the flowers of Asian Buddleja (Matsuda et al, 1995).
The orange pigmentation of the corolla lobes in B.globosa and other New World Buddleja flowers has not been determined, but it could be both a carotenoid-type compound (e.g.: beta-carotene; crocetin) and/or a flavonoid (an aurone). Flavones and carotenoids are virtually ubiquitous in higher plants and certainly present in other tissues of B. globosa (Backhouse et al, 2007). It is predictable that B.x weyeriana 'Golden Glow' has a yellow corolla but with a lilac flush, the former a feature of B. globosa, the latter derived from B. davidii. In the yellow B. x weyeriana hybrids there is a division between the golden yellow of the corolla and the orange of the throat (see fig.1), a similar division seen in most B. davidii cultivars. This makes it more likely that the pigment of the corolla and the throat are chemically different and could suggest the yellow of the corolla is an aurone.
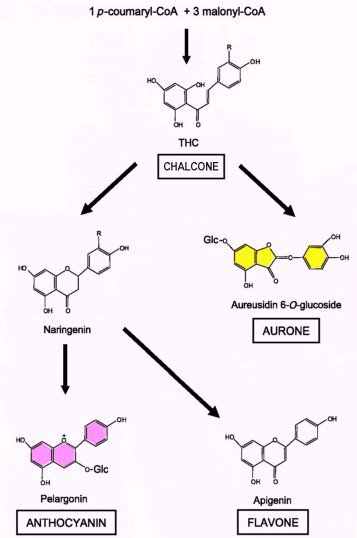
Fig. 4A: Simplified biosynthetic pathway for flavonoid pigments: Flavones, anthocyanins and aurones share common precursors (chalcones, in this example tetrahydroxy-chalcone (THC)). Arrows can represent multiple enzyme steps and the simplest example (i.e.: the fewest hydroxyl substitutions) for each category is shown. (Adapted from Ono et al 2006)
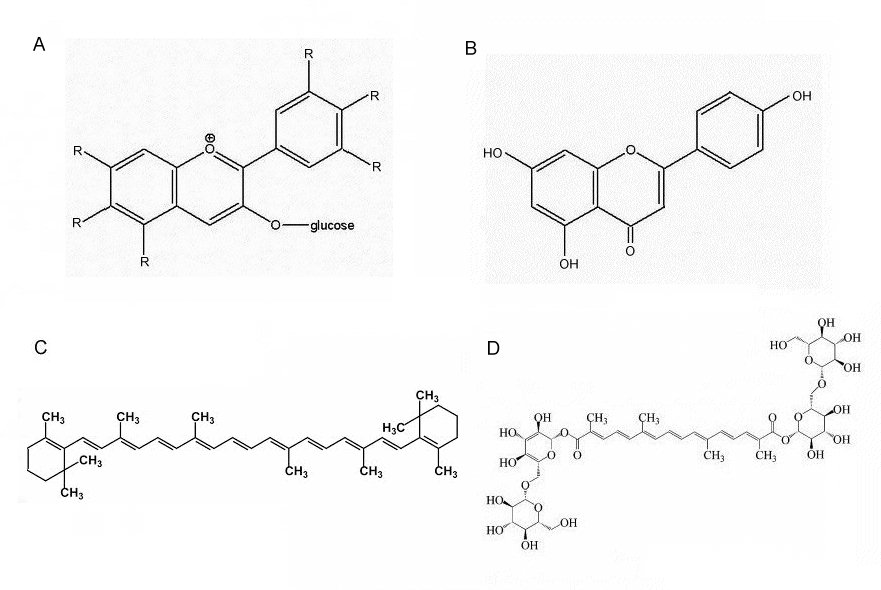
Fig. 4B: A. Anthocyanin (general struture) showing the positions of the side groups (R); B. Apigenin, a flavone found in Buddleja; C. Beta-carotene; and D. Crocetin digentobiose ester.
This lilac colour can be suppressed in B. davidii to produce white-flowered plants. Rather than there being an absence of or fault in the genes that produce anthocyanins, their production is blocked by a gene called Alb2. This is the case in at least one white B.davidii cultivar, 'Nanho Alba' (Tobutt 1993) and probably the same applies to other white or very pale cultivars. I have seen a near white cultivar ('Les Kneale') suddenly produce a dark lilac flower spike where the anthocyanin suppression has failed locally (Fig. 5A). B.x weyeriana 'Sungold' also completely suppresses anthocyanin production in the flower, but this can be restored by loss of effective suppression in rare instances, then the lilac pigment completely masks the yellow in the corolla (Fig. 5B). This is strong evidence for the "suppression hypothesis" of white flower colour.

Fig.5: 'Les Kneale' (A) and 'Sungold' (B) demonstratng the loss of control over anthocyanin production.
Van de Weyer's Original Plants

Fig. 6: 'Golden Glow' was described in Weyer's original article (1920)
The original report of van de Weyer (1920) makes fascinating reading. His B. globosa x B. davidii F1 generation were virtually all greyish white tinted with lilac but for one yellowish-white near-sterile hybrid, and had 'ball-shaped' flowers. He selected one grey-white plant crossed presumably to a sibling or self-pollinated, the latter assuming the self-incompatibility of B. davidii wasn't inherited (Chen et al, 2011), to produce seeds forming his F2. The F2 population was a mixed bag: grey-violet flowers or yellowish flowers in ball-like clusters borne on spikes, as well as a larger number of plants with violet panicles much like B. davidii var. Magnifica. 'Golden Glow'(Fig. 6) was his chosen best plant to exhibit from the former group. He also sowed a F3 generation, but there are no details about these. There are few details about 'Moonlight' (Fig. 7), first exhibited in 1923 (Anon., 1924), but it is most likely from the F2 generation. Other than these two named cultivars, his plants are now lost.

Fig. 7: 'Moonlight' - one of van de Weyer's two original plants.
Genetic Make-up of the Hybrids
It could be assumed that B.x weyeriana would be triploid as the progeny of diploid and tetraploid parent species, however it has been known for some time that it is tetraploid like B. davidii with 76 chromosomes (Moore 1960). It also has a somewhat larger genome that either parent as determined by the DNA content of the cell, being some 26% greater than B.davidii (Van Laere et al, 2009).
Genomic in situ hybridisation (GISH) analysis of the chromosomes from B.x weyeriana 'Sungold' revealed that of the 76 chromosomes 36 were from B. davidii, 28 from B. globosa and 12 recombinant chromosomes (a mix of B. globosa and B. davidii) (van Laere et al, 2010). This nearly equal contribution from each parent suggests that van de Weyer's original plants were the result of so-called unreduced gametes in his B.globosa flowers and the F1 was also tetraploid. Unreduced gametes (B. globosa ovules in this case) have not undergone meiotic reduction and still have the parental complement of chromosomes (2n = 38). These 2n gametes are then more inclined to successful fertilisation by the normal 1n pollen of B.davidii with an equal number of 38 chromosomes, as a mismatch can be a barrier to successful seed formation and germination (van Laere 2008).
Unreduced gametes are well documented in the diploid Asian Buddleja B. lindleyana. These have been demonstrated as pivotal in the breeding of a B. lindleyana x B. davidii hybrid (Elliot et al, 2004) and reciprocal B. davidii x B. lindleyana hybrids (van Laere et al 2008), the latter giving rise to the Buddleja 'Argus' cultivars. However this is not the case with all Buddleja hybrids between species of differing ploidys. A cross of B. davidii and B. asiatica (a tender Asian diploid species) resulted in a triploid, fully sterile hybrid released commercially as Buddleja 'Asian Moon' (Renfro et al 2007).
Recent Efforts to Breed a Yellow B. davidii Hybrid
In order to cross B. globosa and B. davidii successfully it seems likely that one must rely on unreduced gametes being produced in B. globosa, which isn't necessarily a common occurrence. So two separate projects have come to the same conclusion: it would be a good first step to artificially produce a tetraploid B. globosa. In the work of Rose et al (2000) a cultivar CSS18 was chosen for manipulation as it reliably set seed i.e.: it was female. Colchicine (a mitotic-spindle inhibitor) was used to induce polyploidy in nodal section culture and plants micro-propagated. When mature the tetraploid plants obtained had smaller leaves, smaller flowers and shorter internodes than the diploid. Although several crosses were made and later generations were also grown at the East Malling Research Station, Kent (K. Tobutt, personal communication). No further reports have been published, so we don't know how successful the progeny proved to be.
In the work of van Leare (2011) several methods were employed to induce tetraploidy, both in seedlings and shoots, using oryzalin and trifluralin (more potent mitotic inhibitors). Many tetraploid plants were successfully propagated and one was chosen (GLO34) to pollinate B.davidii 'Nanho Alba'. GL034 must have been male as the reciprocal-cross failed and, in any case, the induction of tetraploidy is unlikely to also induce hermaphrodism. It should be noted here that neither report mentions that B. globosa is a dioecious species. The F1 progeny of the cross (Fig. 8a) were rather different from the description of van de Weyer's F1 plants. Of the seven plants raised, five were yellow and two purple with evidence of yellow pigmentation.

Fig. 8a: F1 plants B. davidii x B. globosa (reproduced from Van Laere et al, 2011).

Fig. 8b: Originally yellow-flowered, the lilac pigment has developed as the plant has matured.
The numbers are too few to work out the genetics but certainly support the assertion that 'Nanho Alba' is dominant heterozygous at one locus for anthocyanin suppression (Tobutt 1993). The flowers are less globose that B.x weyeriana 'Sungold', and in interrupted panicles intermediate between the parent species (Fig. 8a).
This breeding programme is no longer active and no further progress has been reported (van Laere, personal communication). I was lucky enough to secure a couple of plants from the programme. I have grown a B. davidii 'Nanho Alba' x B. globosa GLO34B hybrid (B-09-6) for several years and it has proven difficult to establish in the ground. The flowering is quite late compared to B. davdii, early autumn rather than summer, and the flowers can be somewhat meagre. Initially the flowers were yellow, but as it has matured the flowers have developed a lilac pigment. The few seedlings I have managed to raise by its open-pollination have been rather poor specimens, resembling weedy B. davidii, with little evidence for the retention of the yellow-colouring in the outer corolla.
These are not the only reports of artificial chromosome doubling in Buddleja. B. madgascarensis is an African species (section: Nicodemia) with yellow panicles, although not at all cold hardy (Leeuwenberg 1979). It also has potential to be used for the introgression of yellow colour into B. davidii, selecting resulting hybrids with good cold hardiness. A cross of B. madgascarensis x B. crispa, both diploid species, has been made and gave rise to a diploid, sterile hybrid. Sterility precludes further breeding and diploidy limits its application in crosses with B. davidii. Oryzalin was used to induce tetraploidy in the hybrid and fertility was reinstated; these tetraploid plants were successfully crossed with B.davidii 'Nanho Alba' (Dunn and Lindstrom 2007). None of the surviving seedlings of this cross are yellow but they fade to orange/yellow as the flower ages. They are not sterile and the plants are also large which limits their appeal. They are extremely drought tolerant and winter-hardiness has not been an issue (Dunn and Lindstrom, personal communication).
B. x weyeriana X B. davidii Hybrids
Van de Weyer (1920) reported that 'Golden Glow' was not very fertile; however, all the B.x weyeriana cultivars do in fact produce seed. They are practically pollen sterile. B. x weyeriana is readily pollinated by B. davidii. A number of B.x weyeriana x B. davidii hybrids are becoming popular garden varieties: 'Pink Pagoda' and 'Blue Boy' from Peter Moore (Longstock Nursery); 'Bicolor' and 'Attraction' from Mike Dirr (University of Georgia); Flutterby Grande® 'Peach Cobbler' and 'Vanilla' from Peter Podaras (Cornell University). Each one gains its predominat colour from the pollen parent (B. davidii) , but with a slight cast that seems due to a small persistence of yellow pigment. Bicolor's purple colour changes to orange-bronze as the flower fades, due to the partial breakdown of anthocyanins allowing the yellow-orange pigment to show through. Flutterby Grande® 'Vanilla' is off-white retaining almost nothing of the yellow in the outer the coralla.
Van Laere (2008) carried out reciprocal crosses of B. davidii 'Nanho Alba' with B.x weyeriana 'Sungold', managing to extract a small amount of poorly viable Sungold pollen. The seedlings were a mix of pale purple and white, as would be expected from what is known about the genetics of white flower colour (Tobutt, 1993). Sadly, no residual yellow pigment seems to be present in the corolla in the white seedlings from this cross.
I have grown a number of B.x weyeriana x B. davidii seedlings, from open-pollinations in a densely Buddleja-planted garden - they are mixed colours as a result and very variable in many ways. A couple of plants with pale blue flowers show a phenomenon similar to Bicolor in that the blue in the flowers would fade leaving a primrose yellow background (Fig.9), a phenomenon noted by van de Weyer in his F1 plants (Weyer, 1920).
The pigments responsible for the yellow colour of the B.x weyeriana corolla are unknown. The yellow does persist in the corolla of some B. davidii type plants such as in the B.x weyeriana 'Sungold' x B. davidii seedling below (Fig. 9). The potential might be there to retain yellow pigment in the outer corolla and not just the corolla tube, whilst breeding out the lilac/purple anthocyanins (or perhaps more correctly breeding in their suppression), leading to yellow flowers with the inflorescence morphology of B. davidii.

Fig. 9: B.x weyeriana 'Sungold' x B. davidii seedling showing the residual yellow colour in the outer corolla.
The fertility status of the B.x weyeriana x B. davidii hybrids is intriguing. The two Flutterby® plants mentioned above are claimed to be fully pollen and seed sterile, whereas 'Bicolor' produces copious viable pollen but never sets seed (Peter Podaras, personal communication). 'Blue Boy' and 'Pink Pagoda; fail to set seed; whether they produce pollen is unknown. Out of many plants of my own that have reached maturity only one is able to set poorly viable seed, and a couple of others appear to produce seed but these fail to germinate. Most fail to produce seed capsules at all. This raises the possibility that the sex determinants of B. globosa are reasserting themselves in this generation, causing sterility in some and gender-specification in others. This presents a challenge for breeding further generations because at least one parent must be capable of setting seed (either by being female or hermaphrodite) and the most promising plants could well not have this attribute.
Conclusions
Sophisticated and traditional plant breeding efforts have been made to produce the first yellow-flowered Buddleja with the appearance of B. davidii although such a plant has yet to be commercially released or develpoed as of 2023. The tendency to sterility in hybrids may prove a real hindrance in many cases. The full range of orange flowered New World Buddlejas has yet to be utilised in hybridisations with Asiatic plants, as most of them are not common or even known to be in cultivation. Some of these may prove excellent partners for hybridisation with B. davidii: the series Stachyoides is mostly hermaphrodite and there are tetraploid species in other series. Several are high altitude species that may encourage cold hardiness (Norman 2000).
The alternative path to success may be traditional plant breeding with the B.x weyeriana x B. davidii hybrids, but the fertility status of many of these plants is a disadvantage. The potential does appear to be there to breed at the very least a primrose yellow B. davidii-type plant. Also, mutations may arise in
Acknowledgments
My thanks for Katrijn van Laere (Instituut voor Landbouw- en Visserijonderzoek / Institute for Agricultural and Fisheries Research, Belgium) for checking the text for accuracy and to Ann Croft for the photo of the Sungold reversion.
References
Anon. (1924) Extracts from Proceedings of the Royal Horticultural Society (in 'Jounral of the RHS') vol. XLIX, pp. xciii.
Asen, S.; Norris, K. H.; and Stewart, R. N. (1972) Copigmentation of aurone and flavone from petals of Antirrhinum majus. Phytochemistry 11(9): 2739-2741.
Backhouse N, Rosales L, Apablaza C, Goity L, Erazo S, Negrete R, Theodoluz C, Rodriguez J, Delporte C (2007) Analgesic, anti-inflammatory and antioxidant properties of Buddleja globosa, Buddlejaceae. J Ethnopharmacol 116:263-269.
Chen G., Sun H., Sun W., and Norman E. (2011) Buddleja davidii and Buddleja yunnanensis: Exploring features associated with commonness and rarity in Buddleja. Flora 206 Pages 892-895.
De Vogel P. (1967) Keuringen 1966. Dendroflora 4:61.
Dunn B.L. and Lindstrom J.T. (2007) Oryzalin-induced chromosome doubling in Buddleja to facilitate interspecific hybridization. HortScience 42:1326- 1328
Elliott W., Werner D.J. and Fantz, P.R. (2004) A hybrid of Buddleja davidii var. nanhoensis 'Nanho Puprle' and B. lindleyana. HortScience. 39:1581-1583.
Leeuwenberg A.J.M., (1979) The loganiaceae of Africa XVIII. Buddleja L. II. Revision of the African and Asiatic species. Mededelingen van de Landbouwhogeschool. 79:1-163.
Matsuda H., H. Cai H., Kubo M., Tosa H. and Iinuma M. (1995). Study on anti-cataract drugs from natural sources. II. Effects of buddlejae flos on in vitro aldose reductase activity. Biol. Pharm. Bull. 18(3): 463-466.
Moore, R. J. (1960). Cytotaxonomic notes on Buddleia. L. Am. J. Bot. 47:511-517.
Norman E. (2000). Buddlejaceae. Flora Neotropica, Vol. 81. New York Botanical Garden, USA.
Ono E., Fukuchi-Mizutani M., Nakamura N., Fukui Y., Yonekura-Sakakibara K., Yamaguchi M., Nakayama T., Tanaka T., Kusumi T., Tanaka Y.(2006) Yellow flowers generated by expression of the aurone biosynthetic pathway. Proc Natl Acad Sci USA 103:11075-11080.
Renfro, S.E., Burkett B.M., Dunn B.L., and Lindstrom J.T. (2007) 'Asian Moon' Buddleja. HortScience 42:1486-1487.
Rose J.B., Kubba J. and Tobutt K.R. (2000) Induction of tetraploidy in Buddleia globosa Plant Cell Tissue Organ Cult. 63:121-125.
Tallent-Halsell N.G. and Watt M.S. (2009) The invasive Buddleja davidii (Butterfly Bush). Bot. Rev. 75:292-325
Tobutt K.R. (1993) Inheritance of white flower colour and congested growth habit in certain Buddleia progenies. Euphytica 67:231-235.
Van Laere K. (2008) Interspecific hybridisation in woody ornamentals. PhD. Thesis, Faculty of Bioscience Engineering, Ghent University.
Van Laere K., Leus L., Van Huylenbroeck J. and Van Bockstaele E. (2009) Interspecific hybridisation and genome size analysis in Buddleja. Euphytica 166:445-456.
Van Laere K., Khrustaleva L., Van Huylenbroeck J. and Van Bockstaele E. (2010) Application of GISH to characterize woody ornamental hybrids with small genomes and chromosomes. Plant Breeding 129: 442-447.
Van Laere K., Van Huylenbroeck J. and Van Bockstaele E. (2011) Introgression of yellow flower colour in Buddleja davidii by means of polyploidisation and interspecific hybridisation. Horticultural Science 38:96-103.
Van de Weyer W. (1920) Hybrid Buddleias. The Gardeners' Chronicle. ser.3, 68: 181.
Email:
buddlejagarden@gmail.com
© The Buddleja Garden 2011-2025.